Human Factors Guidance from MHRA in the UK (2017) vs. 2016 FDA Guidance for Human Factors
May 23, 2018This summary discusses the following documents:
The Medicines and Healthcare products Regulatory Agency (MHRA) is an Executive Agency of the Department of Health and a government trading fund. They are responsible for using science and research to effectively regulate products (medicines, medical devices, blood and blood components).
In September 2017, the MHRA issued a human factors guidance titled, "Human Factors and Usability Engineering-Guidance for Medical Devices Including Drug-device Combination Products." This guidance is intended for manufacturers of all device classes and developers of medical devices and drug-device combination products to be marketed in the UK and notified bodies responsible for assuring the quality of those devices.
The MHRA guidance is written to be consistent with the FDA Human Factors guidance [1] released on 03 February, 2016 and other international standards, most notably:
In the U.S., the term “ergonomics” is defined as the study of people's efficiency in their working environment and “human factors” is a broader term that additionally encompasses human cognition. Interestingly, in the UK, “Ergonomics” is synonymous with the U.S. term, “Human Factors.” In this document, the term “human factors” is used to encompass other terms such as ergonomics and usability. However, for consistency with other key related documents, the MHRA refers to the process of achieving usable products that address user needs and fit with their practices as "usability engineering."
"Ergonomics (or human factors)" – is the scientific discipline concerned with the understanding of interactions among humans and other elements of a system, and the profession that applies theory, principles, data and methods to design in order to optimize human well-being and overall system performance.
"Use error" – user action or lack of user action while using the medical device that leads to a different result than that intended by the manufacturer or expected by the user.
According to the MHRA guidance, Human factors takes into account features of the intended user population, such as age, size, strength, cognitive ability and training, and intended environment of use, such as hospital wards, intensive care units, ambulances, or home environment; factors such as potential competing distractions, lighting level, or urgency of use will also be considered.
The guidance clarifies that the human factors process is an iterative process, involving design, testing and validation of design stages; it also requires attention to the post-market phase, since evidence may come to light while a device is being used in clinical practice that the design requires further improvement. The aims of a usability engineering process are to deliver products that are easy to use and safe in the intended context of use, and by intended users (whether by care givers or patients themselves). In the figures below, the MHRA guidance maps out the stages of the Usability Engineering Process in more detail.
Not all these techniques are relevant to every process, and other complementary techniques may be applied by people with appropriate expertise; the list of techniques is illustrative rather than mandatory. However, user testing and a use-related risk analysis are normally considered the minimal requirements for human factors.
It is important to note that although the guidance aims to clarify regulatory expectations related to human factors of medical devices marketed in the UK, data other than validation testing could be proposed by applicants to validate safety and efficacy.
Fig 1. Stages of the Usability Engineering/ Human Factors Process (from the MHRA Guidance): The figure below shows the Human Factors process workflow according to the MHRA guidance
![]()
Fig 2. Different stages of the Usability Engineering/Human Factors Process and it’s deliverables: The figure below lists the different stages in the MHRA Human Factors Process, some recommended techniques and information that needs to be provided.
![]()
![]()
Fig 3. Human factors as part of the benefit-risk management throughout the product lifecycle (from the MHRA guidance): This figure emphasizes the importance of product iteration and improvement throughout the life-cycle and highlights the human factors considerations to promote optimal clinical outcome.
![]()
![]()
[1] Food and Drug Administration. (2016). Guidance for Industry and Food and Drug Administration Staff. Applying Human Factors and Usability Engineering to Medical Devices. Food and Drug Administration, Rockville, Md, USA.
︎ Neekole Acorda & Nandini Gurunathan
-
Human Factors Guidance from MHRA for Medical Device Approval in the UK
- 2016 FDA Guidance for Human Factors [1]
Who is the MHRA?
The Medicines and Healthcare products Regulatory Agency (MHRA) is an Executive Agency of the Department of Health and a government trading fund. They are responsible for using science and research to effectively regulate products (medicines, medical devices, blood and blood components).
Purpose of Guidance
In September 2017, the MHRA issued a human factors guidance titled, "Human Factors and Usability Engineering-Guidance for Medical Devices Including Drug-device Combination Products." This guidance is intended for manufacturers of all device classes and developers of medical devices and drug-device combination products to be marketed in the UK and notified bodies responsible for assuring the quality of those devices.
The MHRA guidance is written to be consistent with the FDA Human Factors guidance [1] released on 03 February, 2016 and other international standards, most notably:
-
EN 62366-1:2015 Medical devices, Part 1: Application of usability engineering to medical devices.
-
IEC/TR 62366-2:2016. Medical devices, Part 2: Guidance on the application of usability engineering to medical devices.
-
EN 60601-1-6:2010+A1:2015 Medical electrical equipment, Part 1-6 General requirements for basic safety and essential performance.
-
EN ISO 14971: 2012 Medical devices - Application of risk management to medical devices.
- EN ISO 13485: 2016 Medical devices. Quality management systems. Requirements for regulatory purpose
Key Definitions
In the U.S., the term “ergonomics” is defined as the study of people's efficiency in their working environment and “human factors” is a broader term that additionally encompasses human cognition. Interestingly, in the UK, “Ergonomics” is synonymous with the U.S. term, “Human Factors.” In this document, the term “human factors” is used to encompass other terms such as ergonomics and usability. However, for consistency with other key related documents, the MHRA refers to the process of achieving usable products that address user needs and fit with their practices as "usability engineering."
"Ergonomics (or human factors)" – is the scientific discipline concerned with the understanding of interactions among humans and other elements of a system, and the profession that applies theory, principles, data and methods to design in order to optimize human well-being and overall system performance.
"Use error" – user action or lack of user action while using the medical device that leads to a different result than that intended by the manufacturer or expected by the user.
What is the MHRA Human Factors Process?
According to the MHRA guidance, Human factors takes into account features of the intended user population, such as age, size, strength, cognitive ability and training, and intended environment of use, such as hospital wards, intensive care units, ambulances, or home environment; factors such as potential competing distractions, lighting level, or urgency of use will also be considered.
The guidance clarifies that the human factors process is an iterative process, involving design, testing and validation of design stages; it also requires attention to the post-market phase, since evidence may come to light while a device is being used in clinical practice that the design requires further improvement. The aims of a usability engineering process are to deliver products that are easy to use and safe in the intended context of use, and by intended users (whether by care givers or patients themselves). In the figures below, the MHRA guidance maps out the stages of the Usability Engineering Process in more detail.
Not all these techniques are relevant to every process, and other complementary techniques may be applied by people with appropriate expertise; the list of techniques is illustrative rather than mandatory. However, user testing and a use-related risk analysis are normally considered the minimal requirements for human factors.
It is important to note that although the guidance aims to clarify regulatory expectations related to human factors of medical devices marketed in the UK, data other than validation testing could be proposed by applicants to validate safety and efficacy.
Fig 1. Stages of the Usability Engineering/ Human Factors Process (from the MHRA Guidance): The figure below shows the Human Factors process workflow according to the MHRA guidance

Fig 2. Different stages of the Usability Engineering/Human Factors Process and it’s deliverables: The figure below lists the different stages in the MHRA Human Factors Process, some recommended techniques and information that needs to be provided.
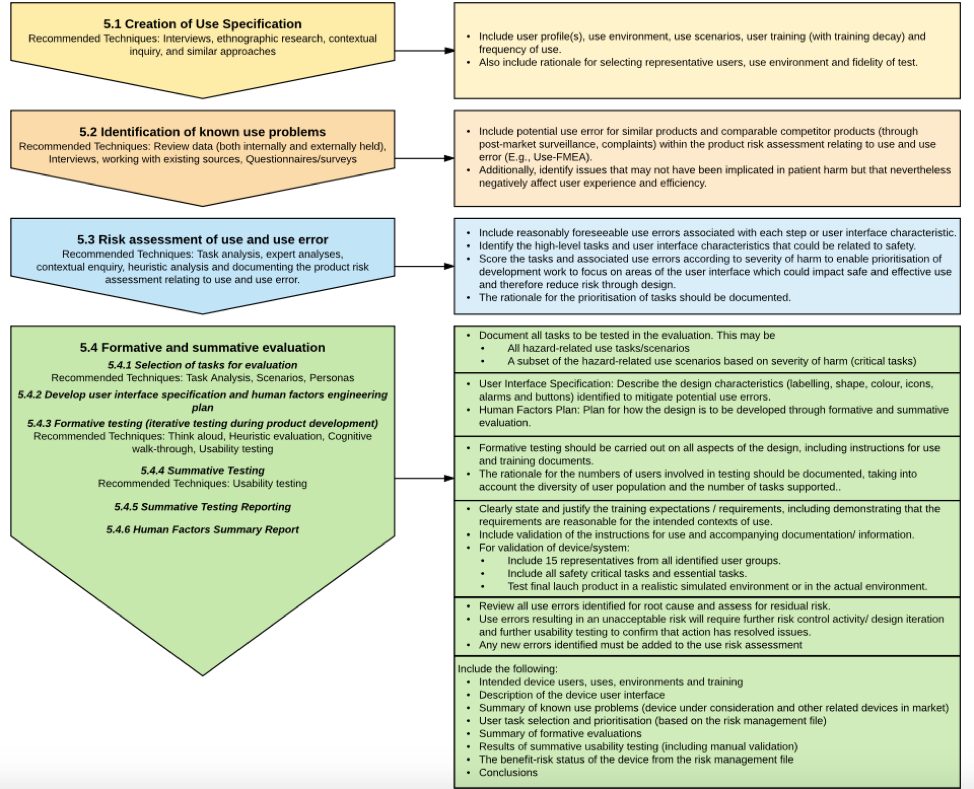
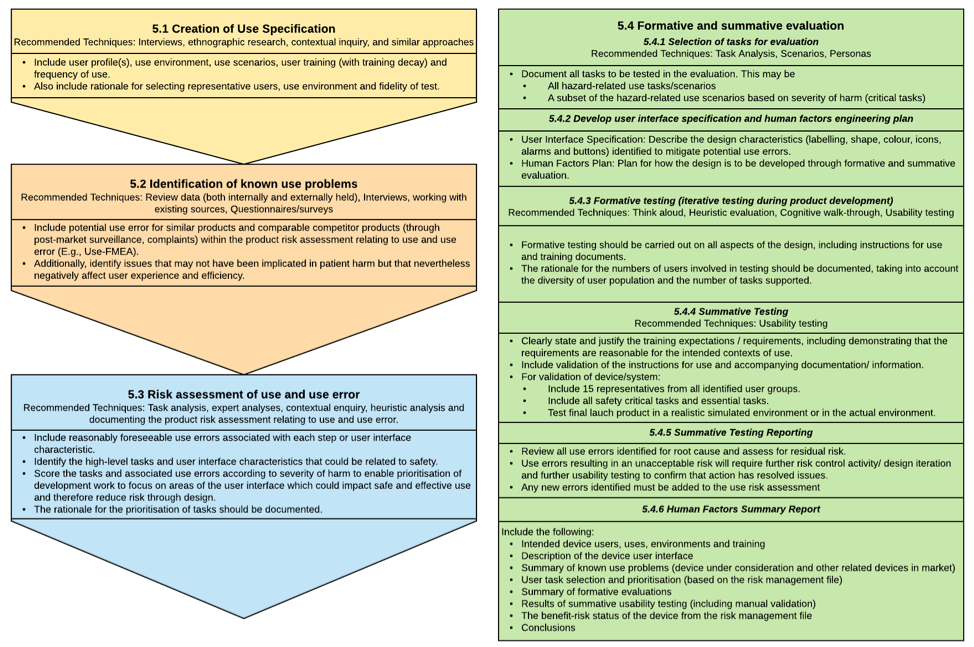
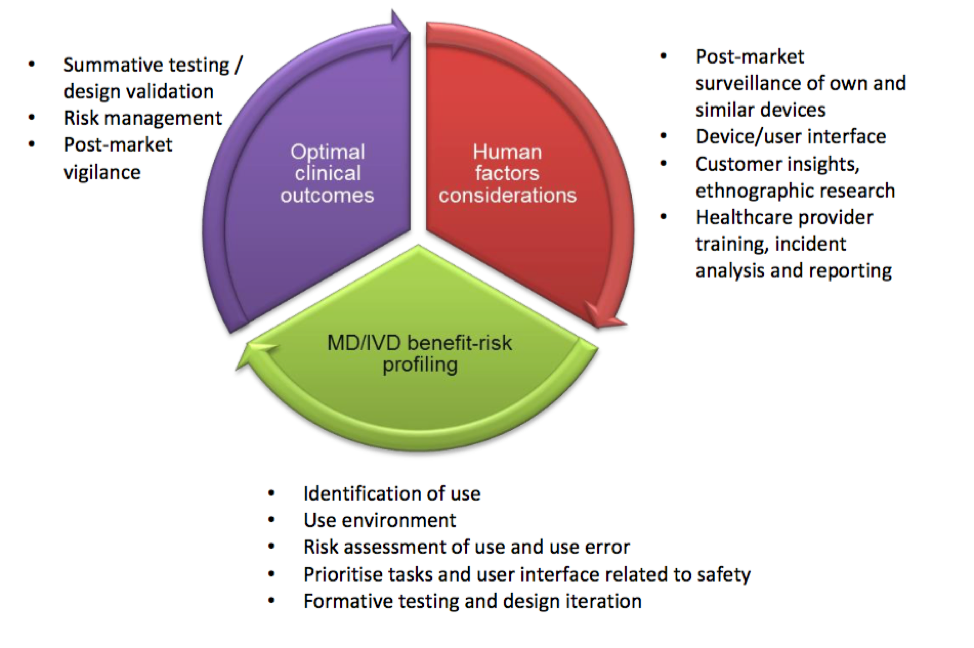
Fig 3. Human factors as part of the benefit-risk management throughout the product lifecycle (from the MHRA guidance): This figure emphasizes the importance of product iteration and improvement throughout the life-cycle and highlights the human factors considerations to promote optimal clinical outcome.
Summary of the MHRA Guidance
-
Increasing international recognition of usability as being essential for ensuring safe use of medical devices
-
Easy to comply with UK Guidance and US FDA Guidance
-
Aims to clarify regulatory expectations related to human factors of medical devices marketed in the UK
-
Guidance is intended for all classes of medical devices, therefore ensuring usability engineering is considered for all medical devices
-
Data other than validation testing could be proposed by applicants to validate safety and efficacy
- Addresses Legacy products (older products that did not have summative human factors testing). The guidance points to following "EN 62366-1:2015 Annex C (normative) 'Requirements for user interface of unknown provenance (UOUP)'” and recommends performing a use-related risk analysis that utilizes post-market data
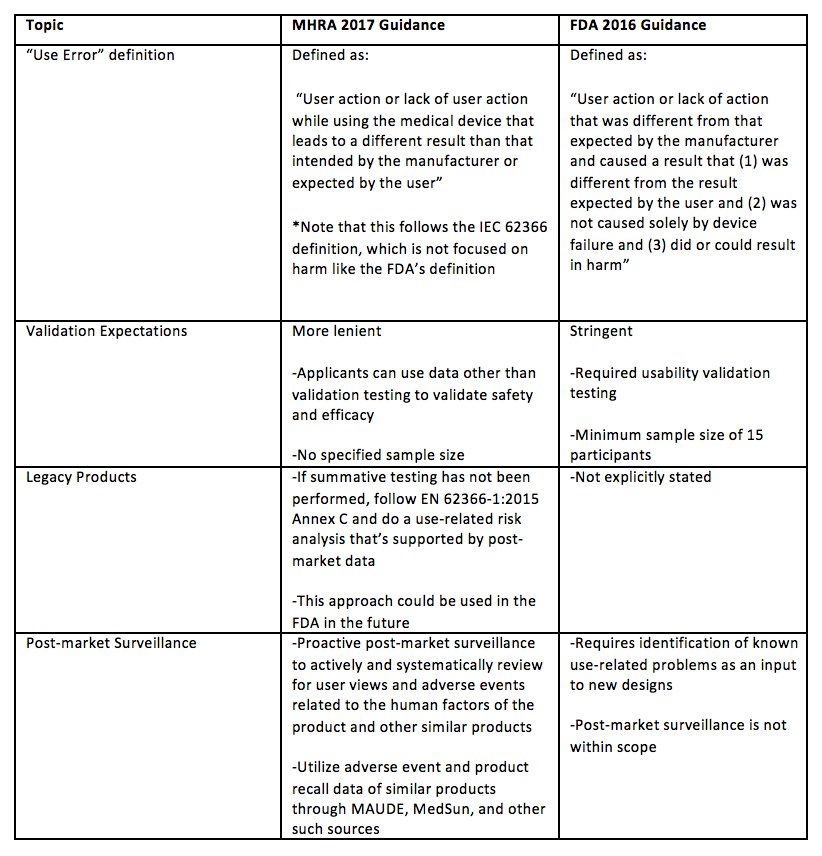
[1] Food and Drug Administration. (2016). Guidance for Industry and Food and Drug Administration Staff. Applying Human Factors and Usability Engineering to Medical Devices. Food and Drug Administration, Rockville, Md, USA.
︎ Neekole Acorda & Nandini Gurunathan
Related Posts


