Improving Complaint Management for Medical Devices Under EUAs with Human Factors

The Emergency Use Authorization (EUA) process is necessary to accelerate the time to market of critical medical products during public health emergencies. However, the lack of design controls associated with this authorization process can lead to improperly capturing and addressing use issues. These design problems may lead to short- or long-term safety, efficacy and customer satisfaction issues. Optimization of complaint-handling systems such that they identify and rectify use-related issues can improve outcomes over the course of the EUA, and help set companies up for success to continue to produce their product after the public health emergency is over. This post details how integration of human factors processes into complaint management systems can improve outcomes for end users and patients.
Introduction
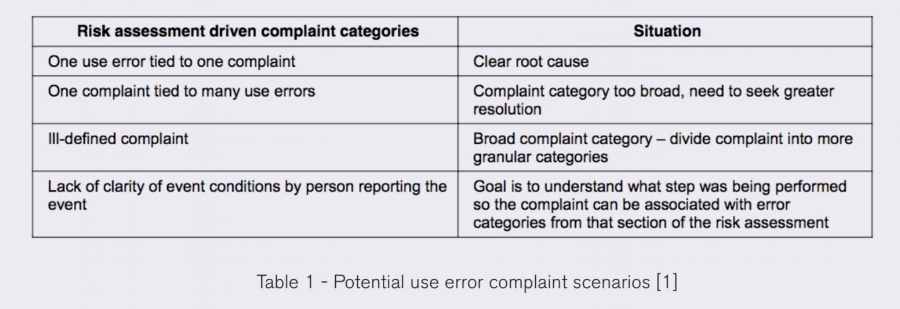
Medical Device Manufacturers need to collect sufficient information and to assign meaningful categories when receiving complaints in order to:
1. Triage complaints in accordance with regulatory requirements
2. Tracking and trending to prioritize and address field issues and potential field issues
3. Perform meaningful root cause analysis of complaints to resolve field issues effectively Medical Device Manufacturers generally have complaint management systems that can robustly analyze product defects unrelated to human error, but complaints related to human error require a slightly different approach.
How can Human Factors best practices help the collection of better data and complaint tracking and trending?
Background
“The Center for Devices and Radiological Health (CDRH) receives approximately 100,000 Medical Device Reports (MDR) in a given year, approximately one- third of which mention “error” on the part of the device users. The nature of these “errors” is not well documented, making the data difficult to analyze and provide for use in new product development.“ [1]
Manufacturers are required by law to investigate and report MDRs and Adverse Events:
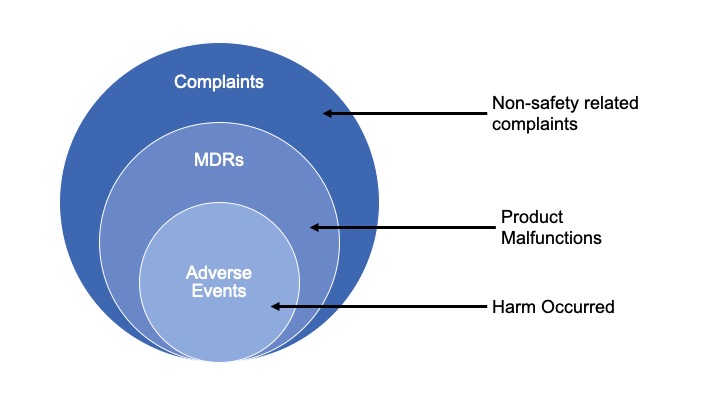
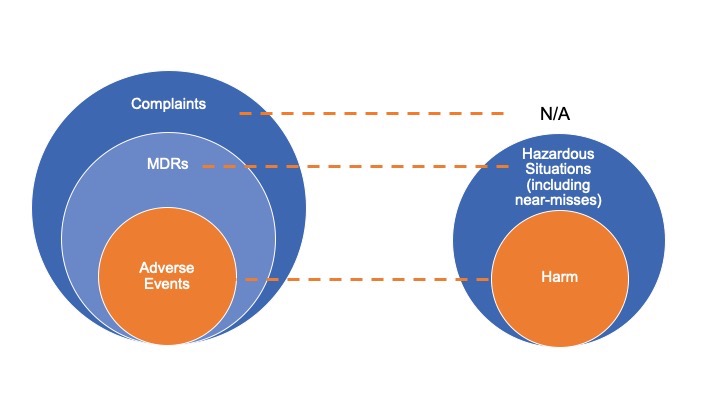
Current State
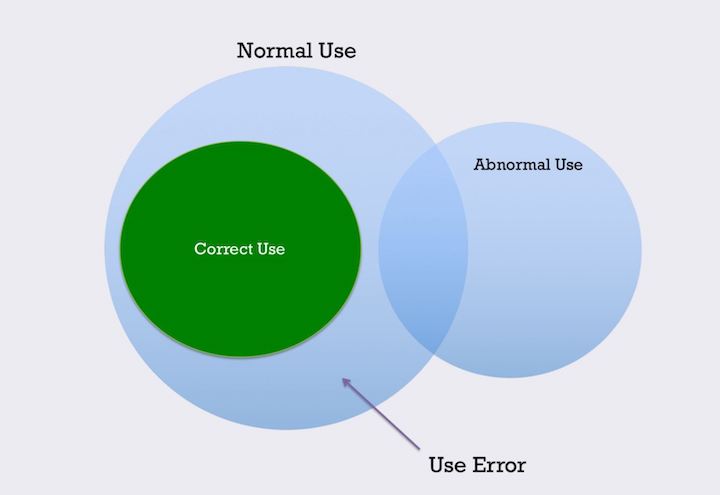
“It is necessary to expand the definition of a “confirmed complaint” to also encompass reproducible use errors, even when there is no evidence of the product physically malfunctioning. As such, the organization must explore what would qualify as a “valid” use event differently than a quality defect complaint. Use error reporting is not a separate reporting process. It is an additional dimension of information that needs to be observed, documented, and reported within the current processes and systems utilized today both within the clinical environment and by manufacturers.” [1]
Future State
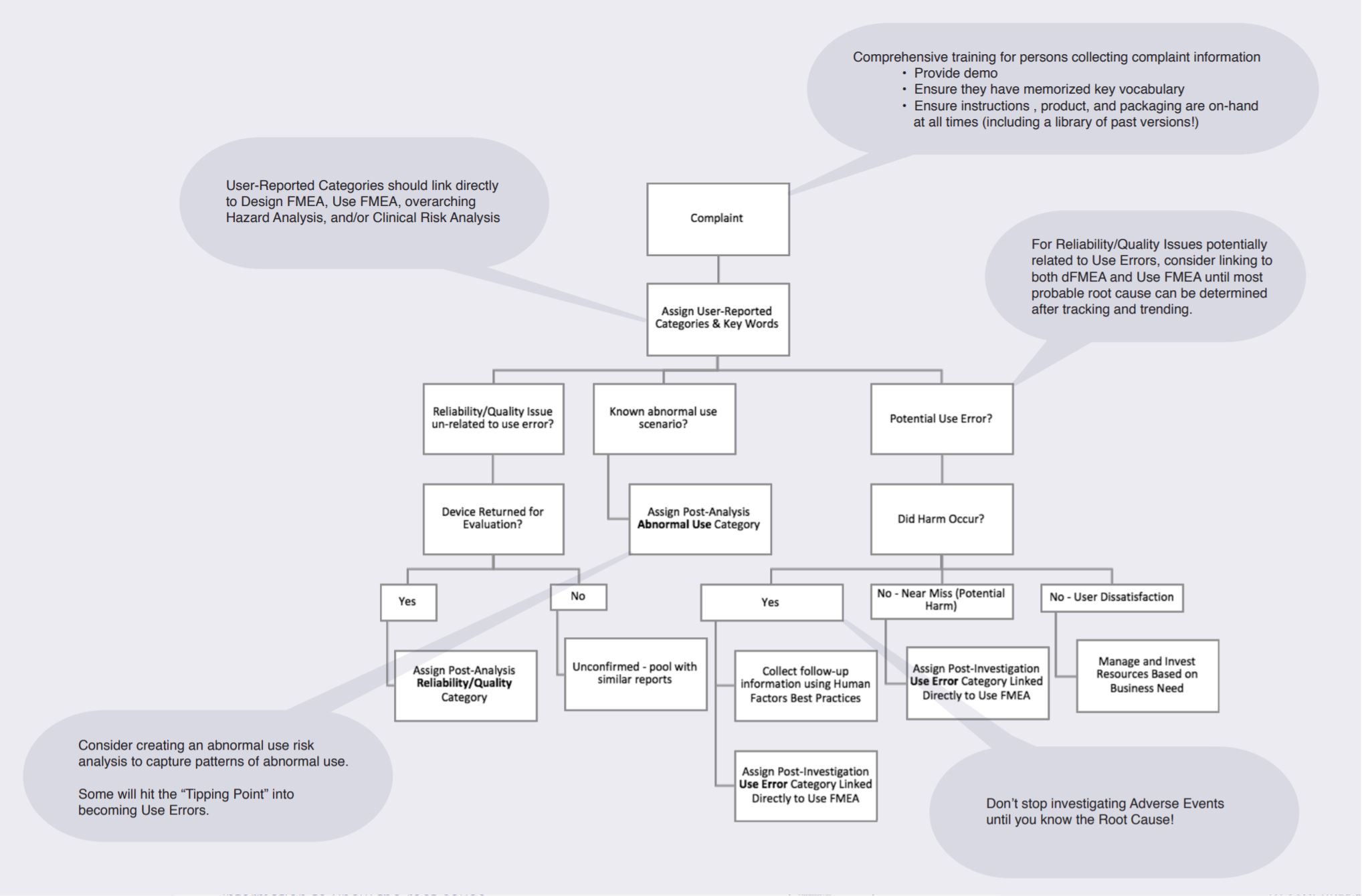
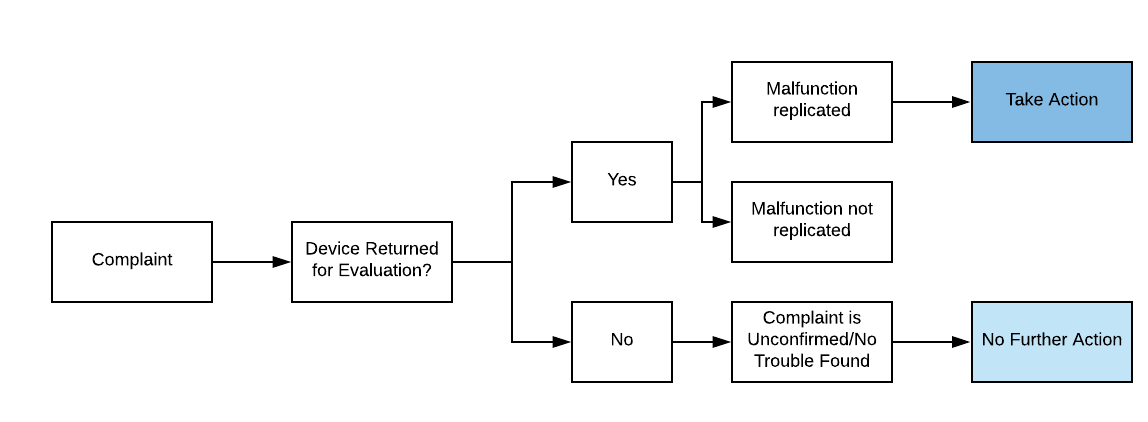
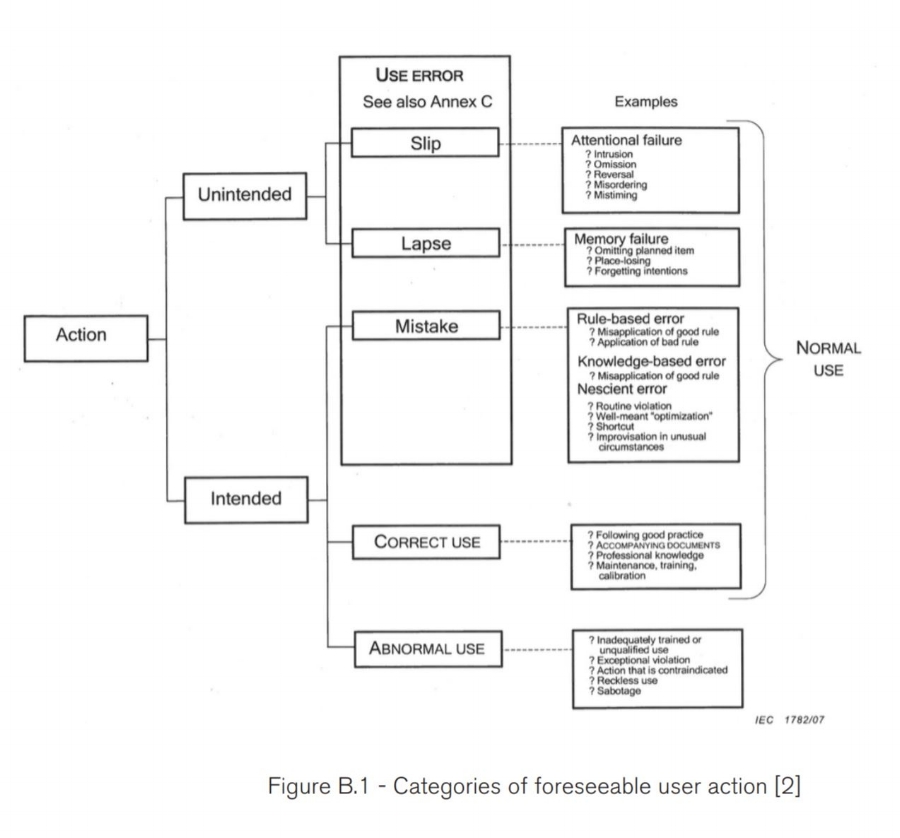
How do I know that I’ve identified Root Cause of a use-related complaint?
If you can identify whether the use error was a slip, lapse, or mistake, then you likely have enough information to know the root cause.
Setting Up Complaints Management System For Success

UserWise Human Factors Process for Complaint Management
1. Link use errors in Use FMEA with complaint categories.
2. Prepare detailed call center scripts with a line of questioning that aligns with human factors best practices.Make sure that the scripts are usable and make sense within existing call center processes.
3. Adequately prepare persons receiving complaints to collect meaningful information. Collecting incorrect or incomplete information can lead to expensive investigations by large teams of engineers
4. Customer service triages complaint with predefined categories. These categories should map to relevant risks and tasks. Allow categories to evolve over time, and be ready for unknown unknowns.
5. MDRs & Adverse Events will be escalated to the quality team for prompt follow-up. Collect supplemental Human Factors Data by sending human factors engineers to the complaint site to ask open ended questions.
Conclusion
Assign Human Factors Engineers to Modernize your Complaint Management System
Save time and money correctly and efficiently identifying root cause of product failures and human error in the field
Close CAPAs instead of being repeatedly haunted by them!
UserWise Can Help Revamp Your Complaint Process
If you are in need of improvement to the complaint handling process at your company, contact UserWise to discuss the next steps for integrating human factors principles into the process.
This is an adaptation of a blog originally posted on December 10, 2018.
References:
[1] AAMI TIR50:2014 /(R)2017 Post market surveillance of use error management
[2] AAMI/IEC 62366:2007 Medical devices — Application of usability engineering to medical devices
︎ Shannon Clark | March 27, 2020
Related Posts


