Medical Device Learnings from the April 2019 IEC Joint Working Group Meeting
In April 2019, Shannon Clark – UserWise founder and CEO – flew to Geneva, Switzerland to participate in the IEC Committee 62A Joint Working Group 4 meeting to further develop and refine international human factors standards. As noted on the IEC website, this committee convenes “to prepare international standards concerning the common aspects of the manufacture, installation and application of electrical equipment used in medical practice, including systems, equipment, accessories, related terminology, concepts, terms, definitions and symbols.” Joint Working Group 4 is focused on usability within this umbrella.
After returning to San Jose, Shannon shared her takeaways from this meeting with the UserWise team, which we have shared below.
She also shared perspectives on the definitions of Normal Use and Abnormal Use. For information about these topics, please see our posts, “Redefining ‘Normal Use’ for Medical Devices,” and a post yet-to-come about Abnormal Use.
This blog post captures three separate topics discussed at the joint working group as IEC 62366-1:2015 was refined and debated, including:
Use by unintended users is not within scope of the human factors process outlined in IEC 62366:2007 nor IEC 62366-1:2015. However, use by unintended users is noted in the U.S. Food and Drug Administration guidance for Human Factors (2016).
Summative usability testing is typically conducted to conclude whether hazard-related use scenarios could foreseeably lead to patient or user harm. The implication of omitting unintended users from the scope of IEC 62366:2007 and IEC 62366-1:2015 is that summative usability testing is not required by these standards to evaluate hazard-related use scenarios related to unintended users.
In contrast, the U.S. Food and Drug Administration may require summative usability testing with 15 lay users to evaluate self-selection of an over-the-counter medical product. Do unintended users understand that they aren’t supposed to use the product, based on the packaging design? The international usability engineering process standards do not require this type of testing.
At the joint working group, it was discussed how unintended users should be considered. Is it important to consider unintended users in the use-related risk analysis? What should drive manufacturers to implement inherent controls to prevent device use by unintended users?
![]() Image credit: Mallery (September 9, 2012). “Baby Needs a Chill Pill.” Horrible Housewife.
Image credit: Mallery (September 9, 2012). “Baby Needs a Chill Pill.” Horrible Housewife.
Ideally, UserWise believes it is important to document use by unintended users in the use-related risk analysis, and that the level of potential risk may determine whether the unintended users should be included in usability validation testing. For example, consider a device meant for smokers. The risk that a non-smoker uses the device should be captured in the use-related risk analysis. If the risk level of this risk is beyond the threshold required by the usability study protocol, then participants in the usability validation study must be tested on their ability to understand the appropriate candidates for the device based on the packaging (i.e., whether a non-smoker should use the device). Even if the risk level is not high enough to be required in the usability validation study, it may be decided to collect the data regardless, for overall usability improvement purposes.
Another interesting question of debate: in which scenarios do patients become users? For example, if an infant is able to pull an IV out of their own body, is the infant a user of the system? Would this scenario then have to be considered in the use-related risk analysis and taken into account when planning the usability validation study?
It is expected that lay users will misinterpret information and act incorrectly from time to time. Many of these errors are “foreseeable” for lay use, though they may be “unforeseeable” for use by medical professionals. As these professionals are trained users, it is expected that their training and expertise will preclude many use errors.
While it is expected that lay users may not follow instructions as thoroughly as professional users are expected to do, culture may determine what manufacturers and human factors engineers should expect of lay users. For example, in the United Kingdom, a usability specialist at the joint working group noted that a higher portion of lay users read the instructions on their own than do lay users in the United States.
When deciding which symbols to use on labeling – the IFU, packaging, and labels – you may choose to use harmonized symbols or to create your own.
![]() Image credit: Schnoll, Les. “World of Confusion: Challenges abound when distributing medical devices globally." Quality Progress.
Image credit: Schnoll, Les. “World of Confusion: Challenges abound when distributing medical devices globally." Quality Progress.
With harmonized symbols, you do not need to test nor explain them. Manufacturers often do explain these symbols (e.g., in the IFU or on a website), to clarify their meaning to the user.
If you create your own symbols, these do need to be tested with representative users. One delegate in the standards committee noted that a symbol is considered internationally acceptable if eighty percent of users can understand the symbol.
If you are looking for help in assembling your human factors or usability engineering plan, UserWise stands ready to help. Our competent team of consultants has years of experience successfully assembling and conducting Human Factors Engineering plans and preparing Human Factors Engineering Submission Reports for the FDA, as well as assembling and conducting Usability Engineering plans and preparing Usability Engineering Files in the EU. Contact us today to learn more about how we can assist in getting your medical product to market.
[1] FDA Guidance Document, 2016. “Applying Human Factors and Usability Engineering to Medical Devices.”
[2] IEC 62366:2007 “Application of usability engineering to medical devices.”
[3] IEC 62366-1:2015 “Part 1: Application of usability engineering to medical devices.”
[4] ISO 15223-1:2016 “Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General Requirements.”
︎ Ali Decker | April 13, 2019
After returning to San Jose, Shannon shared her takeaways from this meeting with the UserWise team, which we have shared below.
She also shared perspectives on the definitions of Normal Use and Abnormal Use. For information about these topics, please see our posts, “Redefining ‘Normal Use’ for Medical Devices,” and a post yet-to-come about Abnormal Use.
This blog post captures three separate topics discussed at the joint working group as IEC 62366-1:2015 was refined and debated, including:
- Use by Unintended Users,
- Use by Lay Users vs. Professional Users, and
- Use of Symbols.
Use by Unintended Users
Use by unintended users is not within scope of the human factors process outlined in IEC 62366:2007 nor IEC 62366-1:2015. However, use by unintended users is noted in the U.S. Food and Drug Administration guidance for Human Factors (2016).
Summative usability testing is typically conducted to conclude whether hazard-related use scenarios could foreseeably lead to patient or user harm. The implication of omitting unintended users from the scope of IEC 62366:2007 and IEC 62366-1:2015 is that summative usability testing is not required by these standards to evaluate hazard-related use scenarios related to unintended users.
In contrast, the U.S. Food and Drug Administration may require summative usability testing with 15 lay users to evaluate self-selection of an over-the-counter medical product. Do unintended users understand that they aren’t supposed to use the product, based on the packaging design? The international usability engineering process standards do not require this type of testing.
At the joint working group, it was discussed how unintended users should be considered. Is it important to consider unintended users in the use-related risk analysis? What should drive manufacturers to implement inherent controls to prevent device use by unintended users?

Ideally, UserWise believes it is important to document use by unintended users in the use-related risk analysis, and that the level of potential risk may determine whether the unintended users should be included in usability validation testing. For example, consider a device meant for smokers. The risk that a non-smoker uses the device should be captured in the use-related risk analysis. If the risk level of this risk is beyond the threshold required by the usability study protocol, then participants in the usability validation study must be tested on their ability to understand the appropriate candidates for the device based on the packaging (i.e., whether a non-smoker should use the device). Even if the risk level is not high enough to be required in the usability validation study, it may be decided to collect the data regardless, for overall usability improvement purposes.
Another interesting question of debate: in which scenarios do patients become users? For example, if an infant is able to pull an IV out of their own body, is the infant a user of the system? Would this scenario then have to be considered in the use-related risk analysis and taken into account when planning the usability validation study?
Lay Use vs. Professional Use
It is expected that lay users will misinterpret information and act incorrectly from time to time. Many of these errors are “foreseeable” for lay use, though they may be “unforeseeable” for use by medical professionals. As these professionals are trained users, it is expected that their training and expertise will preclude many use errors.
While it is expected that lay users may not follow instructions as thoroughly as professional users are expected to do, culture may determine what manufacturers and human factors engineers should expect of lay users. For example, in the United Kingdom, a usability specialist at the joint working group noted that a higher portion of lay users read the instructions on their own than do lay users in the United States.
Use of Symbols
When deciding which symbols to use on labeling – the IFU, packaging, and labels – you may choose to use harmonized symbols or to create your own.
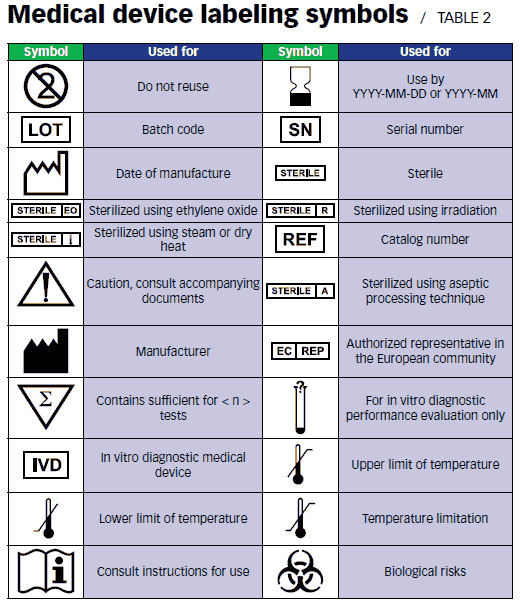
With harmonized symbols, you do not need to test nor explain them. Manufacturers often do explain these symbols (e.g., in the IFU or on a website), to clarify their meaning to the user.
If you create your own symbols, these do need to be tested with representative users. One delegate in the standards committee noted that a symbol is considered internationally acceptable if eighty percent of users can understand the symbol.
Next Steps
If you are looking for help in assembling your human factors or usability engineering plan, UserWise stands ready to help. Our competent team of consultants has years of experience successfully assembling and conducting Human Factors Engineering plans and preparing Human Factors Engineering Submission Reports for the FDA, as well as assembling and conducting Usability Engineering plans and preparing Usability Engineering Files in the EU. Contact us today to learn more about how we can assist in getting your medical product to market.
References
[1] FDA Guidance Document, 2016. “Applying Human Factors and Usability Engineering to Medical Devices.”
[2] IEC 62366:2007 “Application of usability engineering to medical devices.”
[3] IEC 62366-1:2015 “Part 1: Application of usability engineering to medical devices.”
[4] ISO 15223-1:2016 “Symbols to be used with medical device labels, labelling and information to be supplied – Part 1: General Requirements.”
︎ Ali Decker | April 13, 2019
Related Posts


