Human Factors Return on Investment
Through the Lens of Medical Device Recalls

Introduction
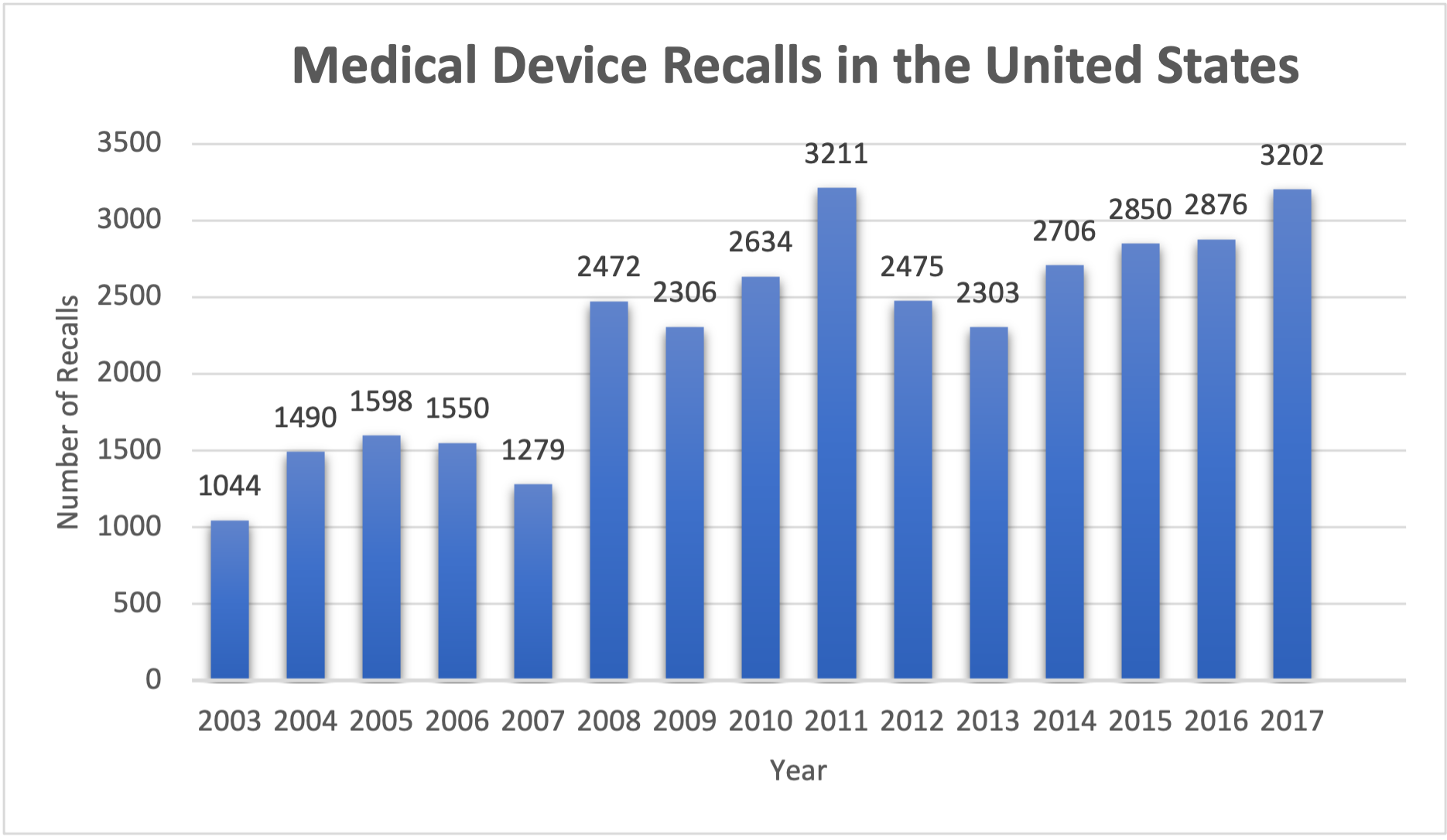
Medical device recalls in the United States have shown a steady rise over the years.1, 2 From 2003 to 2017, the number of medical device recalls in the United States has more than tripled. Additionally, in 2021, there were 55 class I medical device recalls – the most serious type of recall – which is the highest number of class I medical device recalls since 2014.3 The increase in recalls may be attributed to increased medical device complexity, heightened FDA enforcement, and a shift towards more pro-active, safety-focused cultures by medical device manufacturers.
Financial Aspects of Recalls
As of 2015, the cost of a major medical device recall has been as high as $600 million dollars.4 Along with the initial cost of the recall, companies must also allocate funds to product disposal, communication, litigation, and logistics that are associated with the recall.4 An example from the pharmaceutical industry shows that just 35% of the total cost are the direct cost of the recall.6 The majority of the expenses include:
- 49% due to business interruptions,
- 10% resulting from product rehabilitation, and
- another 6% related to extra expenses5

How can Recalls be Avoided?
To better understand how to avoid medical device recalls, it is important to consider the causes.
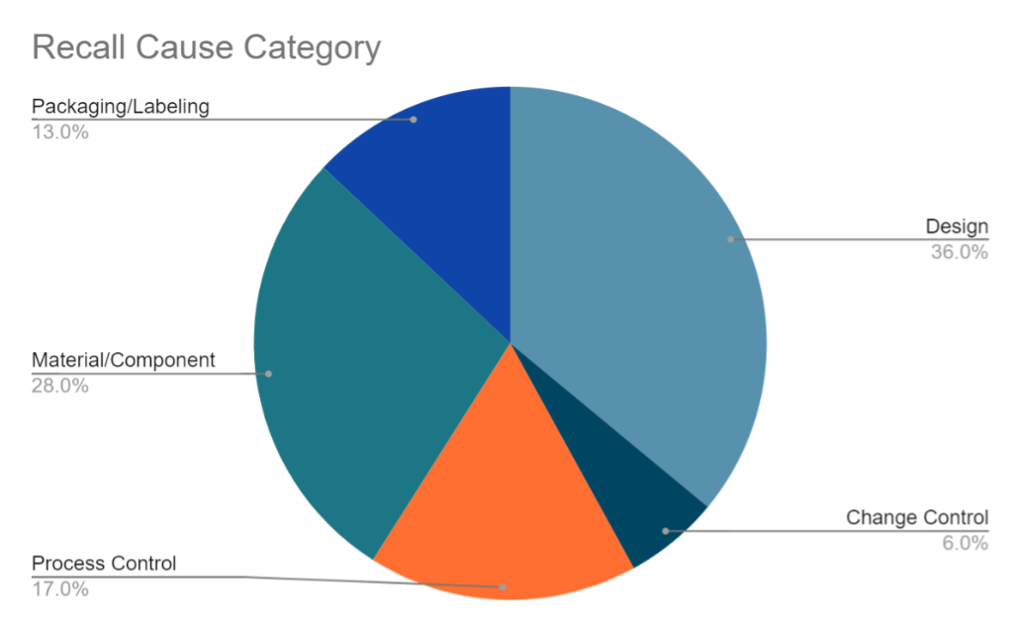
From 2010 to 2012, 36% of medical device recalls were considered the result of a design-related failure.1 Design is the most common cause of recalls, followed by material/component at 28%, process control at 17%, packaging/labeling at 13%, and change control at 6%, as shown in Figure 3.1
To mitigate issues related to device design, Human Factors and Usability Engineering for medical devices has been utilized.6 Potential use errors and task-related risks are uncovered and alleviated using these methods, resulting in design improvement.7 Since recalls are often a direct result of design issues, a reduction in these issues ultimately leads to a decreased risk of product recall.
Human Factors and Usability Engineering provides further long-term financial benefits such as increased sales, user productivity and satisfaction, as well as decreased costs of training, customer support, and maintenance.8 For these reasons, Human Factors and Usability Engineering should be thought of not only as a means to improve user experience through device safety and efficiency, but also as an exceptional return on investment.
Next Steps
If you are interested in earning a significant return on investment for your medical device through Human Factors and Usability Engineering, UserWise stands ready to help. Our team has the skills and expertise to assist you through the entire human factors process in order to mitigate design issues and ultimately reduce the likelihood for a device recall.
- Contact us today to set up a free 1-hour consultation for your project and learn more about our usability engineering
- Check out UserWise’s human factors services.
- Learn more about our usability engineering expertise.
References
[1] Center for Devices and Radiological Health. (2013). Medical Device Recall Report FY2003 to FY 2012, U.S. Food and Drug Administration. https://wayback.archive-it.org/7993/20170404175527/https:/www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHTransparency/UCM388442.pdf
[2] Mezher, M. (2018). Device Recalls in 2017: Making Sense of the Numbers. Regulatory Affairs Professions Society. https://www.raps.org/regulatory-focus%E2%84%A2/news-articles/2018/1/device-recalls-in-2017-making-sense-of-the-numbers
[3] U.S. Food and Drug Administration. (2021). Medical Device Recalls. https://www.fda.gov/medical-devices/medical-device-recalls/2021-medical-device-recalls
[4] Medical Tracking Solutions. (2015). MTS blog: The Big, Bad, and Ugly Costs of Medical Device Recalls. http://medicaltracking.com/about/news-updates/the-big-bad-and-ugly-costs-of-medical-device-recalls
[5] Shewale, S. D., Chavan, M., & Parekh, S. S. (2014). International Pharmaceutical Industry, 6(1), 16-23.
[6] Schroeder, W. (2021). How to Build Medical Device Usability Testing and Validation into your Quality System, FDA Regulations and Medical Device Product and Quality Management System. https://www.greenlight.guru/blog/medical-device-usability
[7] Clark, S. & Israelski, E. (2012). Total Recall: The Consequence of Ignoring Medical Device Usability. User Experience Magazine, 11(1). https://uxpamagazine.org/total-recall/
[8] Karat, C. (1990). Cost-benefit analysis of usability engineering techniques. Proceedings of the Human Factors Society. Orlando, Florida, 839-843.
︎ Dawson Ohligschlager | June 15, 2022
Related Posts


