Usability Engineering for Medical Devices 101
The Usability Engineering Process is used to help design safe, effective, and user-friendly medical devices. This development process is aimed at ultimately reducing the overall risk of the medical device and improving the customer experience and satisfaction. When implemented early in the design process, it can help a medical device company save money and time by identifying potential use errors in the prototyping phase so design controls and mitigations can be implemented effectively. This blog post outlines the critical phases of the Usability Engineering Process and what they entail for a medical device.
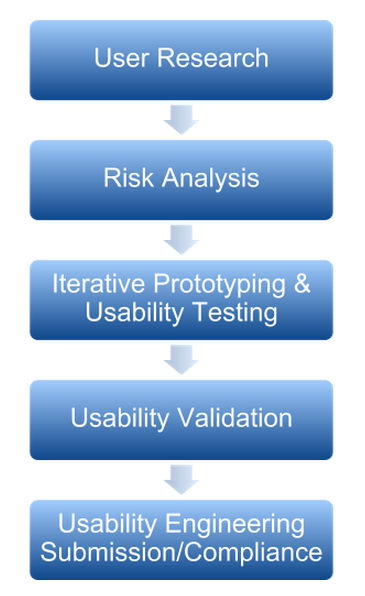
Usability Engineering Process for a Medical Device or Combination Product.
The 5 Parts of the Usability Engineering Process for a Medical Devices & Combination Products
1. User Research
What is Early Stage Human Factors Research?- Collect on-market safety data as an input to the medical device design
-
Find out what people are really thinking through observation at point of medical device use
-
Actions speak louder than words
-
Identify environmental, social, and motivational considerations for the design of the medical device
-
Use appropriate sample, representative of ALL user types
- Ask the right questions
2. Risk Analysis
What is Use-Related Risk Analysis?-
Methodically identify and record all use errors associated with the medical device
-
Use this information to improve the medical device design and maximize safety
-
A Use-Related Risk Analysis is required by regulatory agencies (e.g. FDA)
- Ultimately answer the question, “Is this medical device usable enough to be used by and for my friends and family?”
3. Iterative Prototyping & Usability Testing
What is Iterative Prototyping?-
Create prototypes that are minimally viable for evaluating usability
-
Run many low-cost usability studies to quickly identify and assess use-related risks
-
What is Usability Testing?
-
Behavioral testing to inform design and to identify use-related risks
-
Formally document prototype evaluations to support FDA submission and CE marking
-
Simulate realistic use, without having to release product first
-
“Predict the future” and eliminate surprises in the field
-
Comply with regulations to avoid FDA roadblocks
- The Human Factors branch of the FDA has noted that it’s unlikely to have a successful PMA or 510(k) without performing early-stage usability testing with prototypes
4. Usability Validation
What is Usability Validation?-
Demonstrate the safety and usability of the medical device in simulated use
- Usability Validation is currently required for CE Marking, and a key for successful submission to the FDA for many products
5. Human Factors Submission & Compliance
What is a Usability Engineering Submission?-
For most 510(k)s and ALL PMAs, a Usability Engineering Submission Report is required
-
Including a Usability Engineering Summary Report in a 510(k) can expedite submissions
-
What is Usability Engineering Compliance outside of the US?
-
Compliance to EN 62366:2008 is required in the E.U.
- IEC 62366 compliance includes the need for a Usability Engineering File
Next Steps
Do you need human factors guidance for your medical device or combination product? UserWise is available to help! With a proven track record consulting on more than 150 products to date, an in-house IRB and recruitment team, and contributions as a standards committee member, we are the industry’s premier human factors consultancy.
- Contact us today to set up a free 1-hour consultation for your project.
- Learn more about UserWise’s human factors services.
- Find out more about our unique expertise.
Subscribe to our Newsletter: Sign up for news and updates
︎UserWise, a ClariMed Company | April 26, 2015 [Updated: March 2023]


