Experts Weigh In: Consider Human Factors and Usability Early in Development for Success
UserWise recently participated in an expert panel discussion hosted by the San Francisco Bay Area Biomedical Engineering Society focusing on important questions that companies encounter when trying to apply Human Factors and Usability for Medical Devices. A variety of questions, originating from the audience and from the experts own experience, were deliberated and answered throughout the night. The common theme that emerged from this panel was that human factors is now being recognized as an essential part of the medical device development process. By understanding the human factors process and its nuances, medical device manufactures can be more agile in submitting their medical device to the FDA and Notified Bodies (CE marking), all while improving the safety of their device and ultimate customer satisfaction.
This installment is part 1 in a series of posts on Usability Testing and the Human Factors product submission process. This post addresses the frequently asked question:
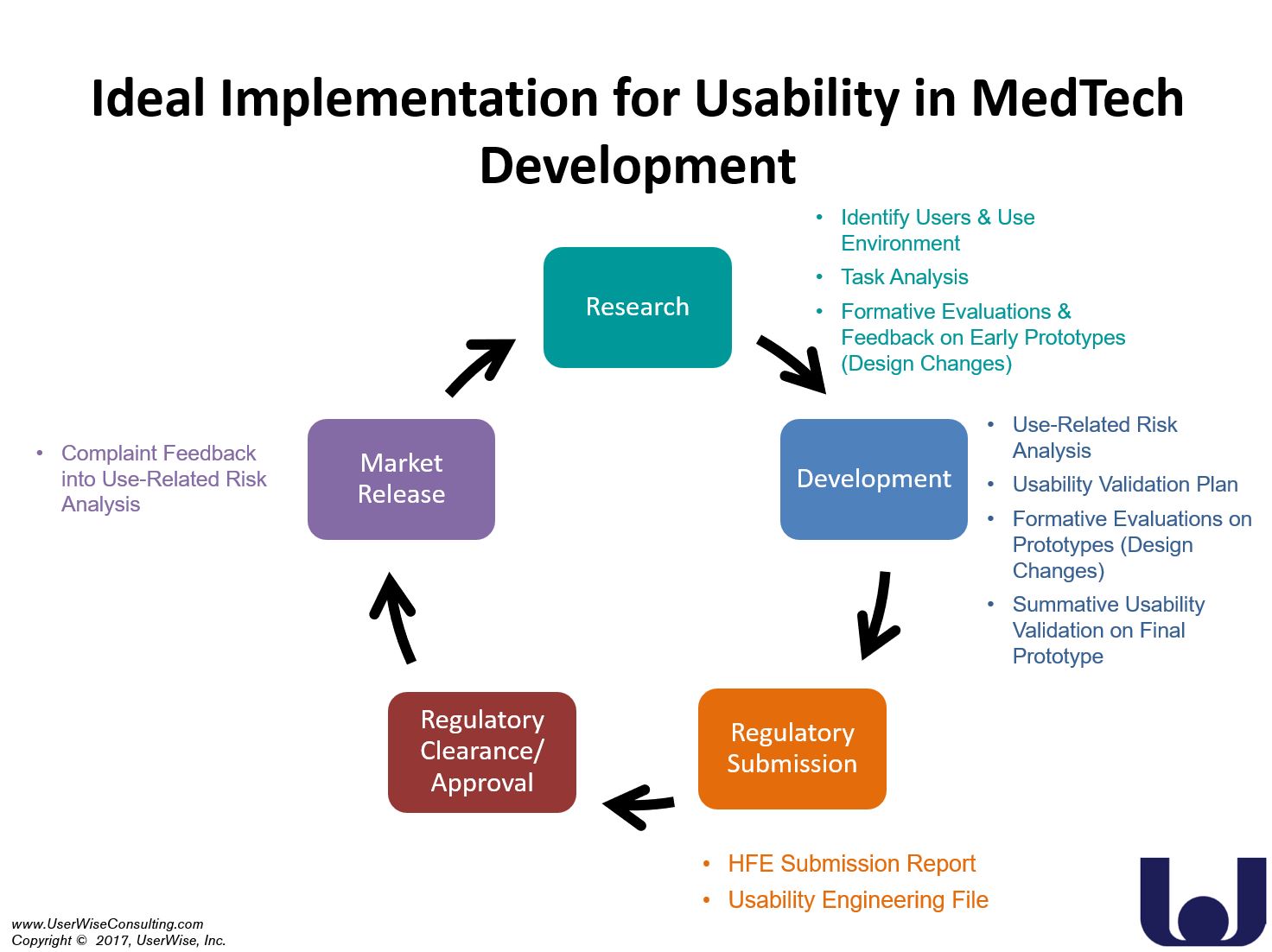
The panelist agreed, in short, the earlier the better. By introducing usability principles early in the product development process, medical device manufacturers are better prepared to tackle usability issues that will inevitably emerge throughout the design process.
Each panelist reiterated the importance of early implementation of human factors and usability in the design process. When implemented early in the design process, usability testing allows for necessary changes to be recognized and implemented. Design changes are much easier to achieve early in development because changes made closer and closer to release require more paperwork and documents to be revised and updated. This can especially be a challenge for the small medical device companies. Having to go back to update large documents or reevaluate tests can cause manufacturers to spend large amounts of time and resources on something that should have been done properly the first time.
![]()
Formative usability testing early in the development process allows for the manufacturer to recognize if the medical device design will work well with intended users. Engineers may think the use of the device is intuitive, but only after the intended users attempt to use the product in the intended use environment will the engineer know if the medical device design is effective. Formative usability testing is a means to troubleshoot what may be needed for the human factors and usability component of the product submission of a medical device. Behaviors of the intended user population may become apparent once a study has been conducted. Unexpected behaviors of the intended user can change the information required for human factors submission to the FDA or notified body.
The panel noted that many medical device manufacturers do not understand all the requirements of the usability engineering process and warned that some companies wait too long before considering usability. The idea was brought forward to create an interdisciplinary Usability Team (e.g., team members from R&D, quality, clinical, etc.) early in the product life cycle to recognize what kind of usability related deliverables will be needed. The Usability Team should continue to meet throughout product development to ensure the input from all team members is accounted for in the human factors process.
Are you interested in learning more about how to apply human factors early in product development cycle for your medical device? We can help. UserWise has proven experience performing gap analyses of existing processes, providing training sessions, and customizing the usability engineering process to fit an individual company’s specific needs. Contact us, we stand ready to assist you.
Be sure to check back soon for the next installment of our series on Usability Testing and the Human Factors product submission process.
︎Garrett Murray & Denise Forkey | November 20, 2017
This installment is part 1 in a series of posts on Usability Testing and the Human Factors product submission process. This post addresses the frequently asked question:
At what point in product development should usability be considered for a medical device?
The panelist agreed, in short, the earlier the better. By introducing usability principles early in the product development process, medical device manufacturers are better prepared to tackle usability issues that will inevitably emerge throughout the design process.
Each panelist reiterated the importance of early implementation of human factors and usability in the design process. When implemented early in the design process, usability testing allows for necessary changes to be recognized and implemented. Design changes are much easier to achieve early in development because changes made closer and closer to release require more paperwork and documents to be revised and updated. This can especially be a challenge for the small medical device companies. Having to go back to update large documents or reevaluate tests can cause manufacturers to spend large amounts of time and resources on something that should have been done properly the first time.
Formative usability testing early in the development process allows for the manufacturer to recognize if the medical device design will work well with intended users. Engineers may think the use of the device is intuitive, but only after the intended users attempt to use the product in the intended use environment will the engineer know if the medical device design is effective. Formative usability testing is a means to troubleshoot what may be needed for the human factors and usability component of the product submission of a medical device. Behaviors of the intended user population may become apparent once a study has been conducted. Unexpected behaviors of the intended user can change the information required for human factors submission to the FDA or notified body.
The panel noted that many medical device manufacturers do not understand all the requirements of the usability engineering process and warned that some companies wait too long before considering usability. The idea was brought forward to create an interdisciplinary Usability Team (e.g., team members from R&D, quality, clinical, etc.) early in the product life cycle to recognize what kind of usability related deliverables will be needed. The Usability Team should continue to meet throughout product development to ensure the input from all team members is accounted for in the human factors process.
Are you interested in learning more about how to apply human factors early in product development cycle for your medical device? We can help. UserWise has proven experience performing gap analyses of existing processes, providing training sessions, and customizing the usability engineering process to fit an individual company’s specific needs. Contact us, we stand ready to assist you.
Be sure to check back soon for the next installment of our series on Usability Testing and the Human Factors product submission process.
︎Garrett Murray & Denise Forkey | November 20, 2017
Related Posts


