IEC 60601-1-6: How Does the New Amendment Impact Human Factors?
Introduction
In July 2020, the International Electrotechnical Commission released the second amendment to IEC 60601-1-6:2010: Medical electrical equipment - Part 1-6: General requirements for basic safety and essential performance - Collateral standard: Usability. This amendment is intended to update the standard to current industry parlance and practice and to align the standard with IEC 62366-1:2015 and ISO 14971:2019. Here, we analyze the important updates that may impact your human factors compliance documentation.
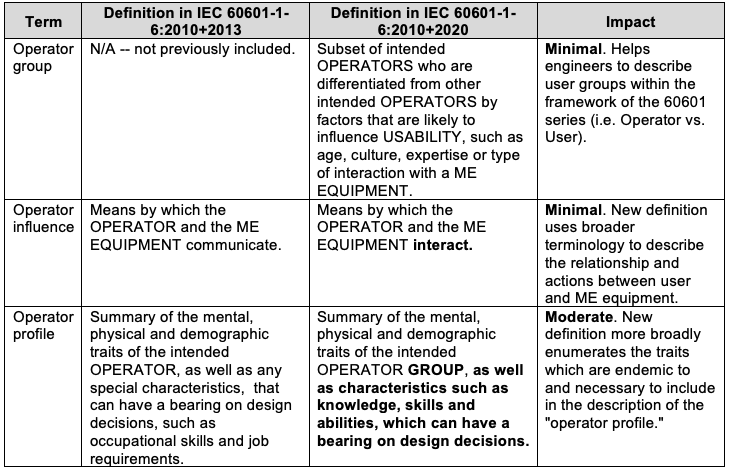
Updated and New Definitions
Important Updates to Relevant Human Factors Activities and Documentation
Updates to General Terminology In Section 4, General Requirements, commonly used terminology is more broadly or accurately defined or contextualized in order to align the standard with the state of industry:
-
"Accompanying documentation" is used instead
of "accompanying documents." This reflects the updated terminology
used in IEC 62366-1:2015.
-
"User group" is considered equivalent to
"operator group." The term, "operator," in the 60601 series
is equivalent to the term, "user," as used in the IEC 62366-1:2015.
-
"Normal use and use error" is referred to as
"normal use, i.e. correct use and use error." This reflects the
currently accepted definition of normal use and, notably, includes use error as
a subset of normal use (see our blog posting on normal use).
-
"Summative evaluation" is used instead of
"usability validation." This aligns the standard with terminology in IEC
62366-1:2015.
-
In the rationale for this section, "use-related
risks" is used instead of "risks associated with usability."
This is indicative of commonly used language in industry.
- "Use specification" is used instead of "application specification." This aligns the standard with terminology in IEC 62366-1:2015. Notably, the requirements for the use specification are distinct from those of the application specification.
-
elements that
require manual manipulation
-
cables and tubing
connections
-
accessories
-
handles
-
force required to
move the weight
-
work surface
height
-
dimensions that
affect reach requirements
-
auditory,
vibratory, tactile, and visual signals to inform OPERATORS
- voice recognition
Updates to Scope of Usability Engineering Process
In Section 2, Scope, the process prescribed by the standard was previously described as a method to "analyze, specify, design, verify, and validate usability." It is now described as a method to "analyze, specify, develop and evaluate the usability." This marks a pivot away from language related to "verification" because verification has been removed as a step in the usability engineering process in IEC 62366-1:2015. Furthermore, the term, “validation,” while preferred by the FDA, has been replaced by the term, “summative evaluation,” in IEC 62366-1:2015.
Updates to Scope of Usability Validation
Section 2 also includes language that impacts the acceptability of the usability of the product. The old language requires that:
-
a) the usability engineering process be complied with
as detailed by the standard and,
-
b) the results of validation testing meet acceptance
criteria laid forth in the Usability Validation Plan,
The amendment to the standard also allows for more flexible methods for summative evaluation. The amendment states that information from post-market surveillance may be leveraged as objective evidence of validation. New standards and regulations, such as the EU's MDR, allow and require for continuous usability improvements and monitoring through the collection and reporting of post-market data.
In IEC 60601-1-6:2010, the rationale for Section 4.1, Conditions for application to ME equipment, stated the purpose of acceptance criteria was to determine "successful validation of the usability of the primary operating functions." New language from the amendment refers to the criteria for risk acceptability as helping to determine "that the operator interface can be used safely." This marks a shift from the language of IEC 62366:2007 to that of IEC 62366-1:2015. With the updates to these standards, it is clearer that a risk-based approach should be taken, and not a quantitative approach, when determining the acceptability of a user interface with respect to usability.
How does this impact the Human Factors process?
This amendment is predominantly focused on bringing the standard's language and rationale up-to-date with the current state of industry. If your usability engineering program is in compliance with IEC 62366-1:2015, you will likely be aligned with this amendment. The most notable changes to the standard were made to the description of the user interface and the definition of operator profile. If your product falls under the purview of this standard, make sure that early usability engineering activities include adequately defining the user profiles and user interface as outlined in this amendment.
References:
IEC 60601-1-6:2010+2013+2020
IEC 62366-1:2015
ISO 14971:2019
︎ Miles Buroker | December 8, 2020
Related Posts


